ExpressPlex™ 2.0 Library Prep Kit
ExpressPlex 2.0: Workflow simplicity plus the performance of TnX for high throughput plasmid and amplicon sequencing.
- Reduced insertion bias and improved coverage uniformity
- One-step fragmentation, barcoding, and amplification
- Auto-normalization
- 96- or 384-well plate format
- Up to 6144 index combinations
We’ve unleashed TnX, our next generation transposase, in ExpressPlex 2.0 – a library prep kit that changes your NGS workflow forever!
ExpressPlex 2.0 is an automation-friendly, one-step library prep powered by TnX to reduce bias and improve coverage uniformity using a highly streamlined workflow for sequencing synthetic constructs like plasmids & amplicons.
Recover More Data From Your Sequencing
Simply spike-in PhiRx™ to increase your index color-balancing!
PhiRx Indexed Control NOW included with every ExpressPlex Library Prep Kit
Advancing Therapeutic Antibody Development

RECORDED WEBINAR
Moderna presentation – Antibody Sequencing: New Operating Model
In this presentation, Nasthas Lacerda Almeida from Moderna, highlights how her organization has leveraged ExpressPlex™ 2.0 for a clonal antibody sequencing approach, optimized for speed and scalability using de novo assembly of NGS short reads.

APPLICATION NOTE
Advances in Plasmid Verification: The Role of NGS in Identity & Clonality Testing.
This application note compares ‘gold-standard’ Sanger sequencing with next-generation sequencing (NGS) for plasmid QC focusing on their strengths, weaknesses, and performance metrics particularly for detecting contaminant constructs that can arise from co-transformation events.
TnX Transposase: Quality Libraries for Quality Sequencing
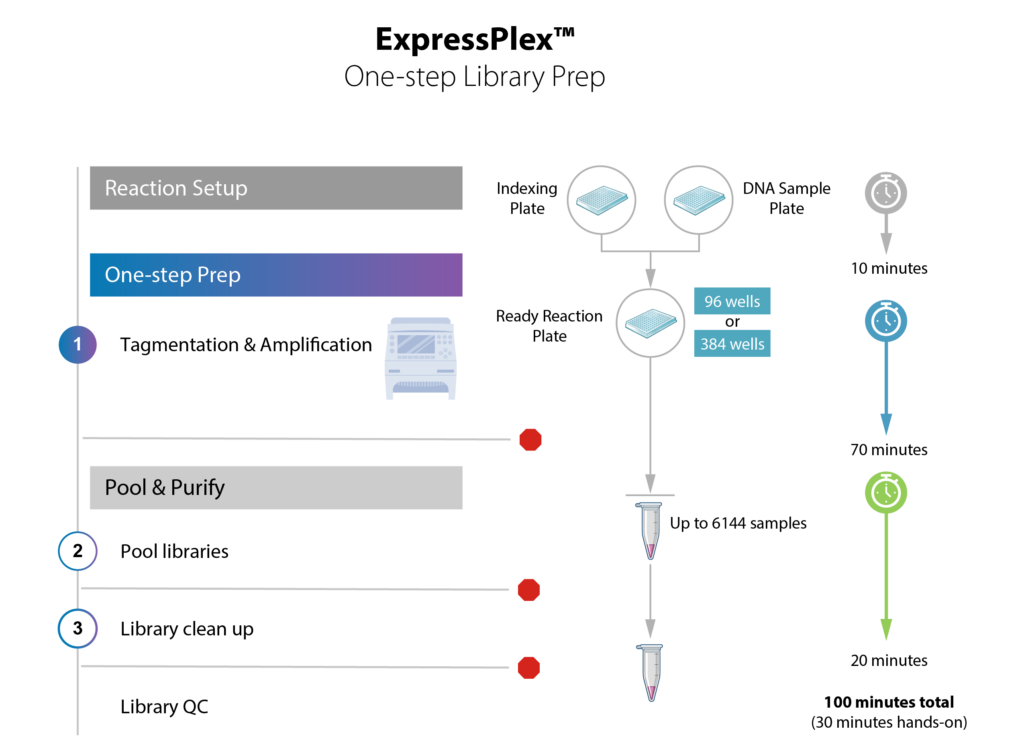
ExpressPlex 2.0 uses tagmentation powered by TnX, the next generation transposase, to simultaneously fragment & index sample DNA. Average fragment size ranges from 400-1200 bp.
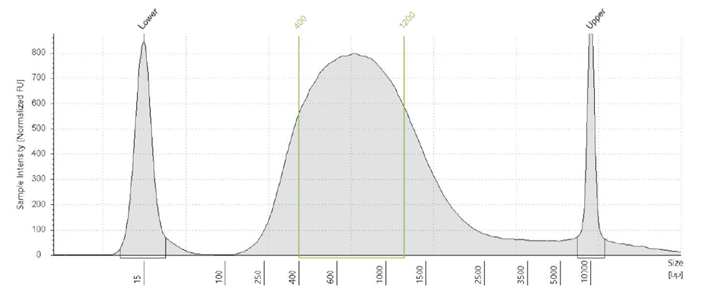
Typical fragment profile following analysis on an Agilent TapeStation System. Data shown are for a 96-plex sample.
TnX: Transposase Engineered for Reduced Insertion Bias
Better transposase = better sequencing. Take your plasmid and amplicon screening to the next level!
ExpressPlex 2.0 is the only library prep kit powered by TnX! TnX was engineered to reduce insertional bias to improve uniformity of coverage. When directly compared to a Tn5-based competitor library prep kit for sequencing of 2 different plasmids, ExpressPlex 2.0 produced significantly lower CVs and range of coverage (max/min).
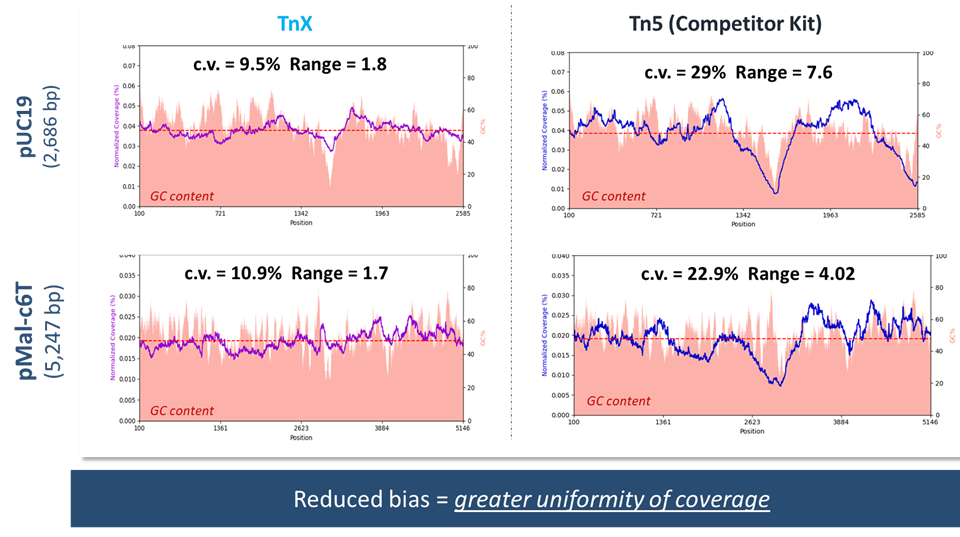
Library preparation for pUC19 and pMal-c67 was conducted using ExpressPlex 2.0 kit and a Tn5-based competitor kit using standard manufacturers’ protocols. Libraries were sequenced using an Illumina MiSeq.
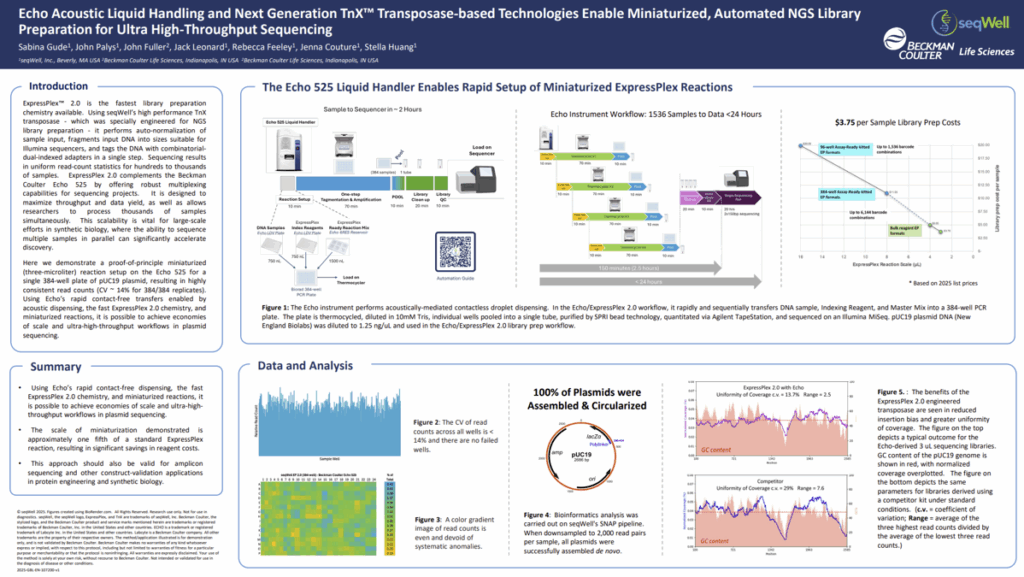
POSTER: Echo Acoustic Liquid Handling and Next Generation TnX Transposase-based Technologies
Enable Miniaturized, Automated NGS Library Preparation for Ultra High-Throughput Sequencing
View Now
Product Highlights
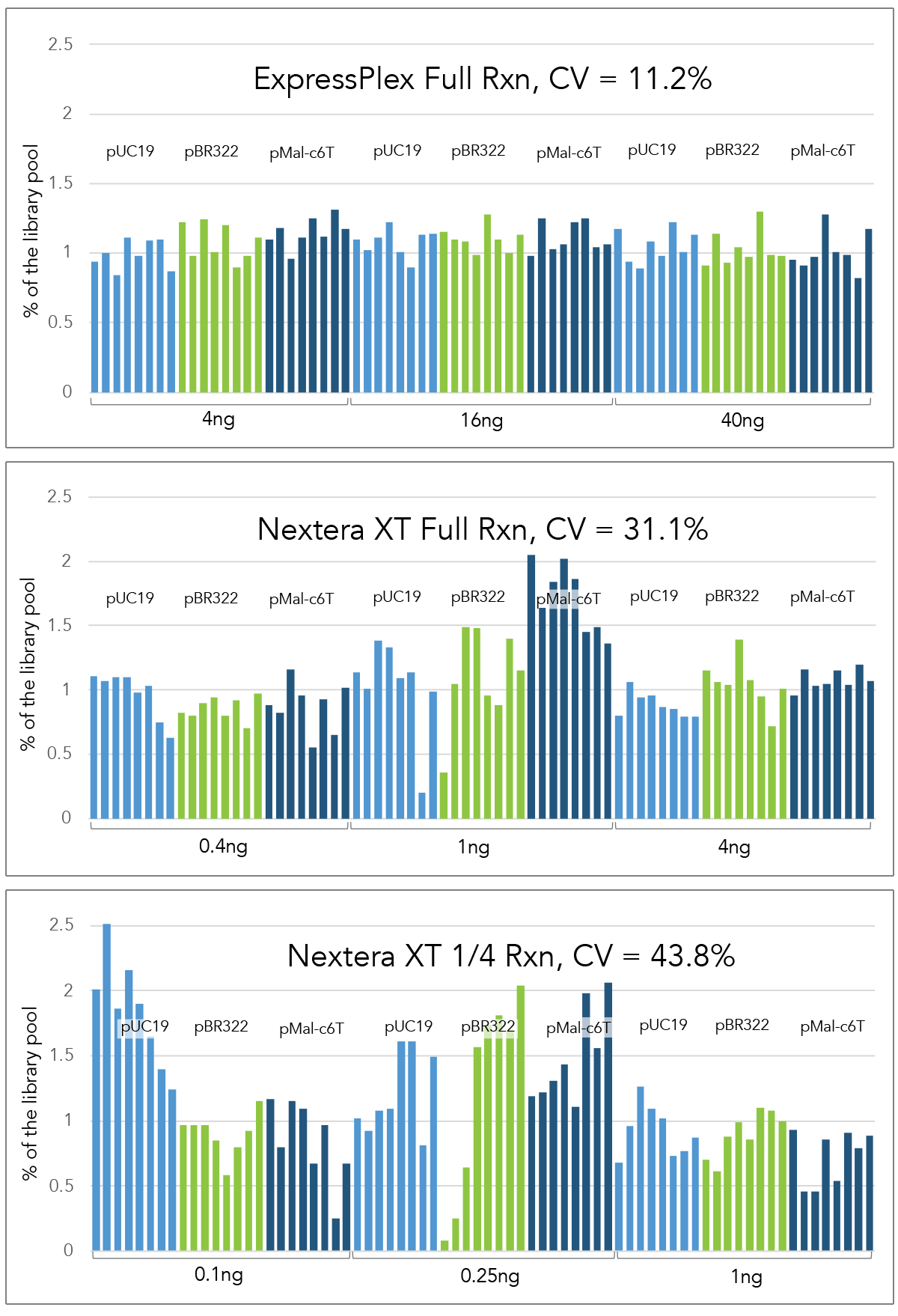
Auto-Normalization of Read Depth
ExpressPlex demonstrates significantly higher levels of normalization compared to competitors, enabling a simplified workflow where individual normalization is no longer required to achieve more consistent read-depths across samples.
Libraries were prepared from three different reference plasmids of varying sizes across a 10-fold input range at full reaction volume for ExpressPlex (16 µL) and Nextera XT (50 µL), as well as at ¼ miniaturized reaction volume for Nextera XT.
For each method, inputs ranged from 4-40ng for ExpressPlex (standard input is 16ng) and 0.4 – 4ng for Nextera XT (standard input is 1ng). Inputs were scaled appropriately for 1/4 reactions.
Auto-Normalization of Insert Size
ExpressPlex generates highly consistent library insert sizes over a broad range of DNA inputs.
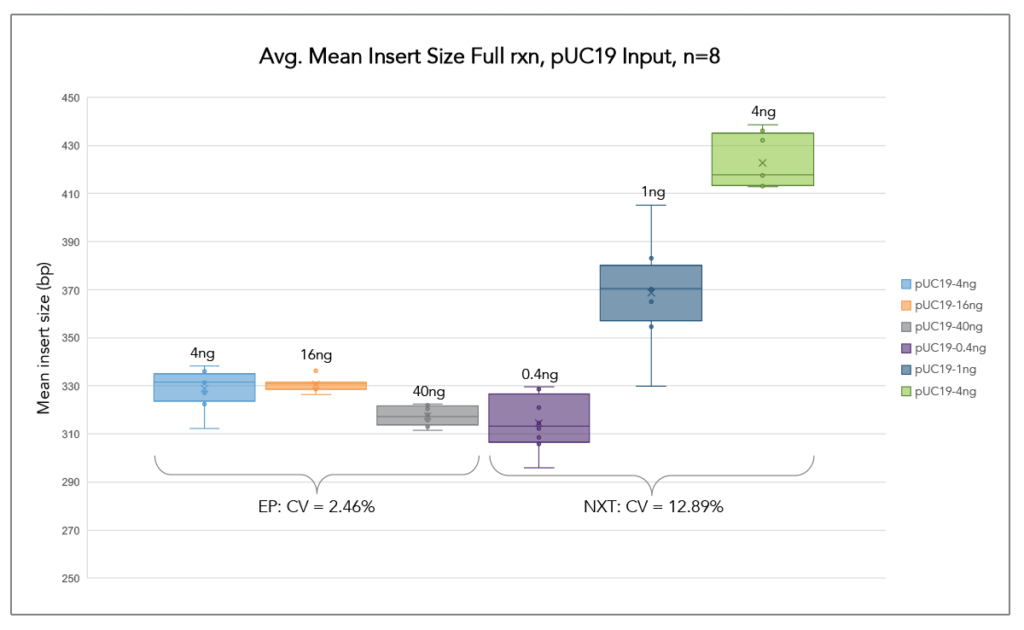
Libraries from pUC19 plasmid DNA were simultaneously prepared at 3 different input amounts (n=8 each input amount). ExpressPlex demonstrated greater consistency in average insert size across a 10-fold range. In each case, the standard input for both kits (16ng for ExpressPlex, 1ng for Nextera XT) was also included as a mid-range data point.
ExpressPlex 2.0: 96-well workflow
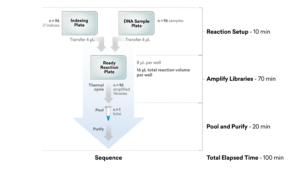
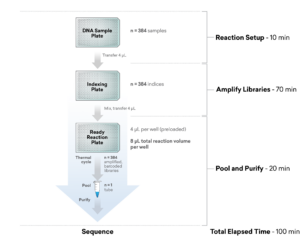
ExpressPlex 2.0: 384-well workflow

Recorded Webinar | Octant Bio
The game-changing capabilities enabled by the ExpressPlex workflow were highlighted in a webinar collaboration with the synthetic biology team from Octant Bio, a drug discovery company located in Emeryville, CA. Octant’s Henry Chan, PhD (Synthetic Biology Lead) and Bryan Jiang (Research Associate) discussed the application of ExpressPlex to OCTOPUS, the high-throughput plasmid sequencing platform that powers Octant’s therapeutic discovery platform. Watch the video to discover how ExpressPlex has dramatically improved OCTOPUS and accelerated their overall discovery process.
Improving Sustainability Through Streamlined Protocols

The automation-friendly ExpressPlex one-step tagmentation workflow enables a >80% reduction in tips/plastics usage. We help drive sustainability not only in our internal sequencing services pipeline, but for our customers across the globe.
Are you in need of bulk-dispensed product for your miniaturized reactions?
ExpressPlex 2.0 Specifications
| Primary Applications | Plasmid & amplicon sequencing |
| Sample Input types | Purified plasmid DNA, RCA-amplified DNA, amplicons >350bp, and colony PCR amplicons |
| Transposase | TnX – Next generation engineered transposase |
| Kit format |
|
| DNA Input Recommended | 96-well format:
384-well format:
|
| Total Library Prep Time | 100 minutes (30 minutes hands-on time) |
| Indexing Method | Combinatorial Dual Indexing |
| Number of Unique Index Combinations | Up to 6144 |
| Batch Size |
|
| Output Fragment Range* | 400 – 1,200bp |
| Number of PCR Cycles |
|
| Sequencer Compatibility |
|
* Fragment size will depend on magnetic bead cleanup ratios used.
ExpressPlex 2.0 Library Prep Kit Includes:
- Indexing Reaction Plate(s)
- Ready Reaction Mix Plate(s)
- MAGwise Paramagnetic Beads
- PhiRx Indexed Control
- Users do not need to supply polymerase, primers, or barcodes. These are contained within the supplied ready reaction mix.




