Plasmid Sequencing Services
Request Services Today
Plasmid sequencing as soon as next business day
Get the power of NGS with the speed of Sanger
Our fast plasmid sequencing service allows you to scale your research without the costly investment in resources and equipment. We perform all whole-plate library preparation, sequencing and data processing on site. Our sample submission process is simple and our fastest turn-around time is next business day*. Your data is delivered directly to you through a secure web server for easy access.
Best value
![]()
Best value on services for 96 well plates
data next day
![]()
Fastest high-throughput turnaround for plasmids
quality data
![]()
Illumina-based sequencing for the highest quality data
Here’s what you get with our plasmid sequencing services:
-
Fast turnaround: Data to you as soon as next business day*
-
Library prep using our ExpressPlex™ Prep Kit
-
Illumina 2×150 sequencing
-
Plasmid de novo assembly via seqWell’s SNAP pipeline and/or FASTQ file delivery
*Plates must be received by 12pm eastern for next day turnaround. Assumes successful sequencing run, turnaround not guaranteed.
Timeline to Your Data

Step 1: Plate Received
Your plate submission in 96 or 384-well format is received by noon Eastern Time.

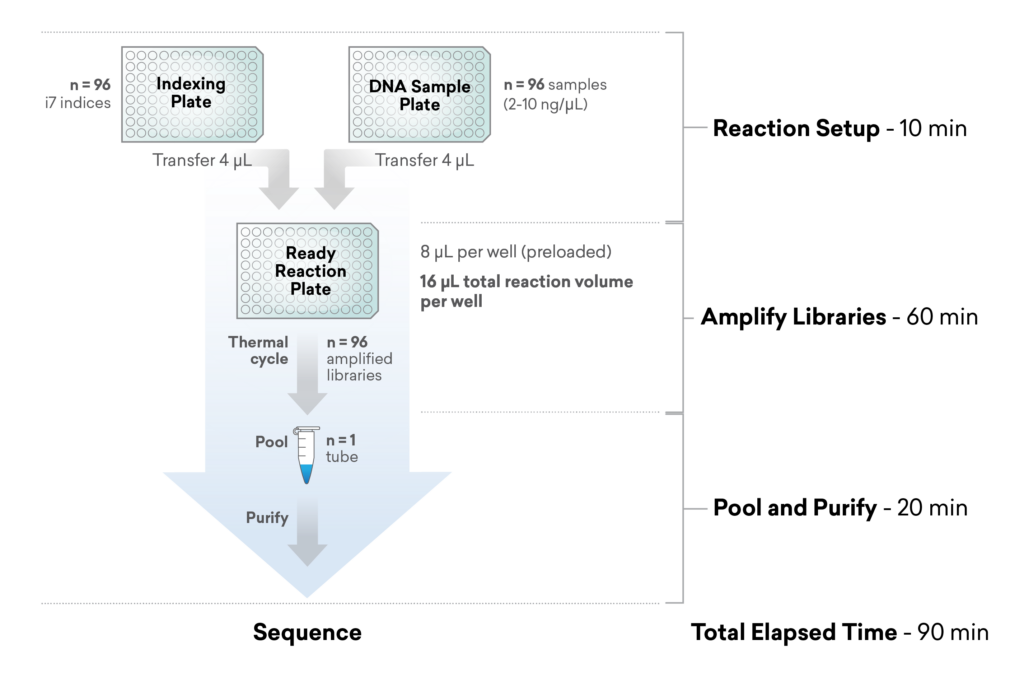
Step 2: Library Preparation
Samples to sequence-ready libraries in 90 minutes using ExpressPlex™ Library Prep
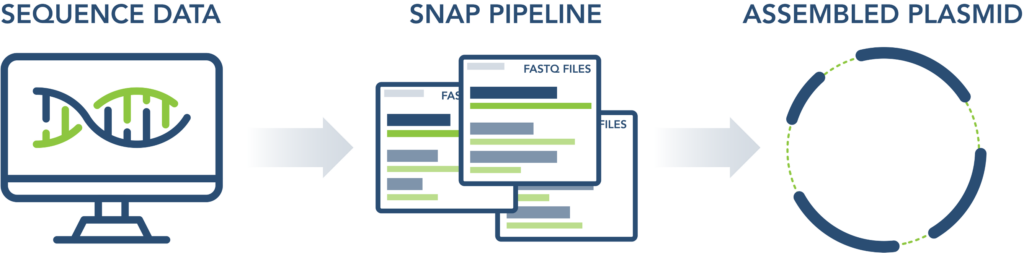
Step 3: Sequencing Run
Data delivered by end of next business day with de-novo assembly via custom analysis pipeline (SNAP).
Data delivery features and options:
- Either circularized, de novo assembled plasmids via our SNAP pipeline, or
- FASTQ and/or FASTA files delivered via electronic AWS Link for your proprietary pipeline
Fast, consistent, reliable service with ExpressPlex™
The introduction of ExpressPlex, our newest library prep kit, into our service pipeline, has changed the game when it comes to workflow simplification.
The one-step workflow enables efficiency and scalability.
- Extracted sample to libraries on sequencer in <1/2 a day
- Easy to automate or train any lab technician, minimizing chance for error
- Suitable for bulk or custom format dispensing, enables scalability & batch size flexibility.
- Reaction volume flexibility achieves desired economics & scale.
- Significant cost & plastics savings over other protocols.
Depend on our NGS experts
Our team of sequencing specialists has extensive NGS experience and has been performing fully-assembled plasmid sequencing for clients for almost a decade. Let our experts do the work so you can spend your valuable time on discovery and analysis, not sequencing.
Sample Submission Guidelines:
- Concentration of all samples between 10 and 100 ng/µL (as measured by dsDNA fluorescent assay)
- Circular plasmids up to 20 kb in length
- Submit samples in plates
- Submit sample submission form and shipping tracking number to services@seqwell.com
- Plates received by 12 pm eastern will be processed same day
Plasmid Sequencing & Assembly Resources
Sample Submission Guidelines
Sample Submission Form
Let us know how we can help you scale
Complete the form below to request more information about our plasmid sequencing services.
