Introduction
The high-throughput management of biologics is increasingly important in the drug discovery process, with the crucial need to automate plasmid production. The steps toward plasmid production are well known from making a brand new plasmid, or retrieving one from storage, to transformation, culture media inoculation, colony picking, culture expansion, and plasmid purification.1 To guarantee accuracy, plasmid DNA sequencing must be confirmed, introducing the further steps to prepare the plasmids for sequencing and analyzing the data.
The question then becomes, can each of these steps be automated and still maintain quality, and what does that automation look like?
The Pros of Automation
The key to successful automation is reliable, effective, and quality processes that eliminate the errors found in tedious manual production while greatly enhancing multiple volume runs. Some of the hallmarks of successful automation are:
- Improved sample tracking
- Reproducibility
- Increased throughput
The manual process to prepare sufficient quantities of plasmid DNA for downstream processes is time-consuming, physically difficult, and prone to errors, especially as sample throughput increases. Through the optimization of several modules, the implementation of an automated high-throughput plasmid DNA production pipeline is achievable.2
Automation of a complex process is not without its challenges. Each manual step must be optimized for the automation platform and may include optimization of the upstream and downstream steps as well. For example, researchers in a 2016 study found that colony picker efficiency was increased to 99% by optimizing the upstream transformation process.2
Challenges Facing High-Throughput Plasmid Production
The management of plasmid-based reagents is particularly challenging and unique. Unlike compound management where the samples are managed via dilution and liquid transfers of bulk synthesized materials, plasmid-based reagents are regenerated/expanded as requested within the sample management facility.
As stated in the introduction, plasmid sequencing has multiple steps, and automation technologies are specifically designed to optimize whatever step they are involved in. So, it’s no surprise that one of the greatest challenges to automation is the multiple steps in the plasmid DNA production pipeline, each of which is necessary to automate. At any point along this automation process, a breakdown in quality could result and render the test results useless.
Additionally, all parts of the automation need to be optimized to work together effectively and deviations from the manual process must be validated to ensure quality does not suffer.
For example, when purifying plasmid DNA manually, there is very little delay between each process step. On an automation platform, samples may sit on the deck throughout the robotic run. This delay could pose a challenge to quality. However, through multiple tests, it has been proven that the stability of the purified plasmid was not affected even when allowed to sit for 7–16 hours.2
Steps such as transformation, inoculation, and colony picking are largely unavoidable and run linearly. However, latter steps such as culture expansion and purification can be done in parallel with sequencing to confirm the plasmid sequencing earlier in the process. This is often achieved through rolling circle amplification (RCA) from the same colonies used in culture expansion.3
Following this pathway, the RCA product can be used as input into NGS library preparation and sequencing. This strategy allows for parallel, automatable processes to both verify plasmid sequence and generate the purified plasmid.
A Dynamic Development
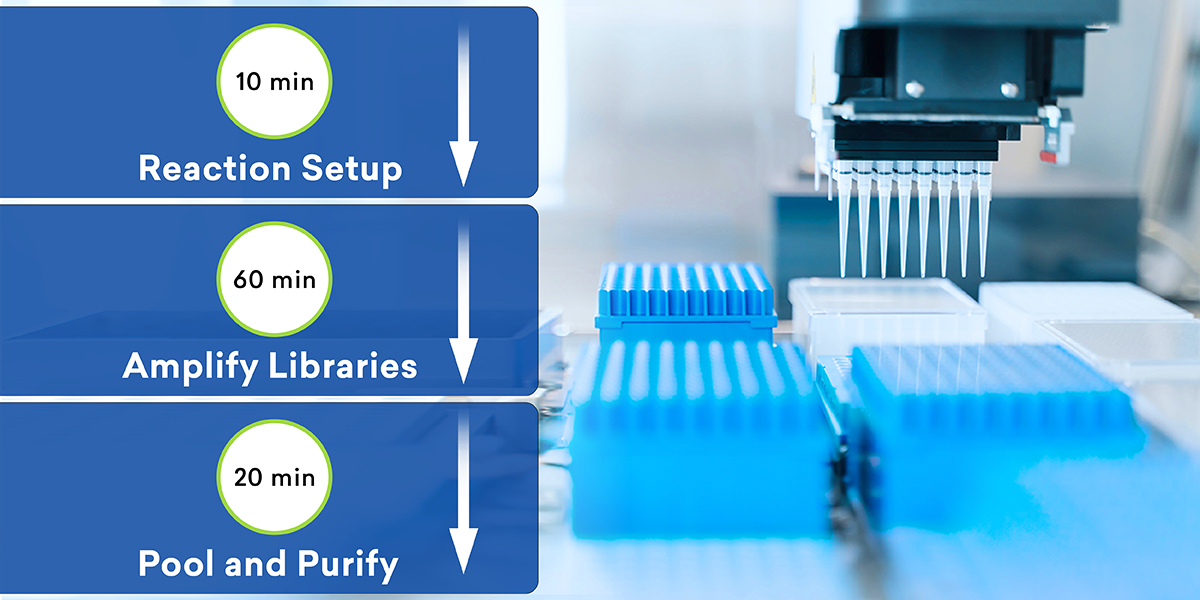
Conventional library preparation methods demand multiple incubations interrupted by cumbersome pipetting steps. seqwell’s ExpressPlex™ transformational library prep technology is our solution.
ExpressPlex can automate protocols by cutting workflows to 90 minutes. The simplicity of this workflow makes it uniquely well-suited for manual, automated, and miniaturized library prep from plasmids and PCR products.4
What’s more, ExpressPlex is compatible with RCA, which means you can multiplex hundreds of samples quickly and easily, freeing up your time to focus on data and results.
Conclusion
Our work at seqWell never stops as we continue to develop ways to improve the quality and scale of NGS-based plasmid sequencing pipelines.1 ExpressPlex greatly cuts workflow times allowing you to spend your time on data and results—the goal of researchers everywhere who are working on the frontline of the drug discovery process.