Introduction
Plasmids are a fundamental research tool and building block for molecular biology and genetic modification of cells. These tiny pieces of DNA are easy to work with and can be manipulated in a variety of ways, making them essential for research in areas such as synthetic biology, cell and gene therapy and functional genomics assays. Plasmids are core to cutting-edge applications of genetic engineering, such as CRISPR-based genome editing.
Increased access to cost-effective, massively parallel next-generation sequencing (NGS), as well as advances in DNA synthesis and synthetic biology, have revolutionized the scale on which plasmids can be constructed, screened, and manipulated in a wide range of applications, including biomarker discover, antibody engineering, genome editing and gene therapy.1 NGS platforms now allow researchers to routinely characterize complete plasmids or similar synthetic DNA constructs in just hours—a feat that would previously take days or weeks using legacy methods such as Sanger-based sequencing and primer-walking.
Challenges in Plasmid Prepping
Preparing plasmid templates for DNA sequencing can be one of the most time-consuming steps in the sequencing process. Current template prep methods rely on labor-intensive, multistep procedures that take up to 24 hours and produce templates of varying quality and quantity.2
So-called minipreps are still the gold standard for preparation of high-quality amounts of plasmid DNA for downstream processing and sequencing. Minipreps involve the use of enzymes to break open bacterial cells used to propagate plasmids and release the plasmid DNA, which is then purified using alcohol precipitation. This purified DNA can be used for many downstream applications, such as PCR or sequencing. Having higher-quality DNA for a downstream sequencing process will usually minimize the risk of errors or artifacts in the resulting data. The most common methods of plasmid DNA preparation are solid phase reversible immobilization (SPRI) bead technology, commercial plasmid kits or homemade recipes. Yet, all of these methods rely on overnight growth of bacterial hosts to amplify the amount of cloned DNA. 2
While mini-preps are a routine method for producing template plasmid DNA for sequencing reactions, the method can sometimes result in DNA that has variable or insufficient quantity for sequencing, depending on factors such culture growth or plasmid copy number. In addition, mini preps can be difficult to scale up to larger starting material amounts, which can be necessary for some applications. As a result, there is a need for improved methods for DNA isolation that can yield high-quality DNA samples in sufficient quantity for sequencing, especially for research applications that require large numbers of different plasmid samples.
RCA: A Simple Yet Effective Technique
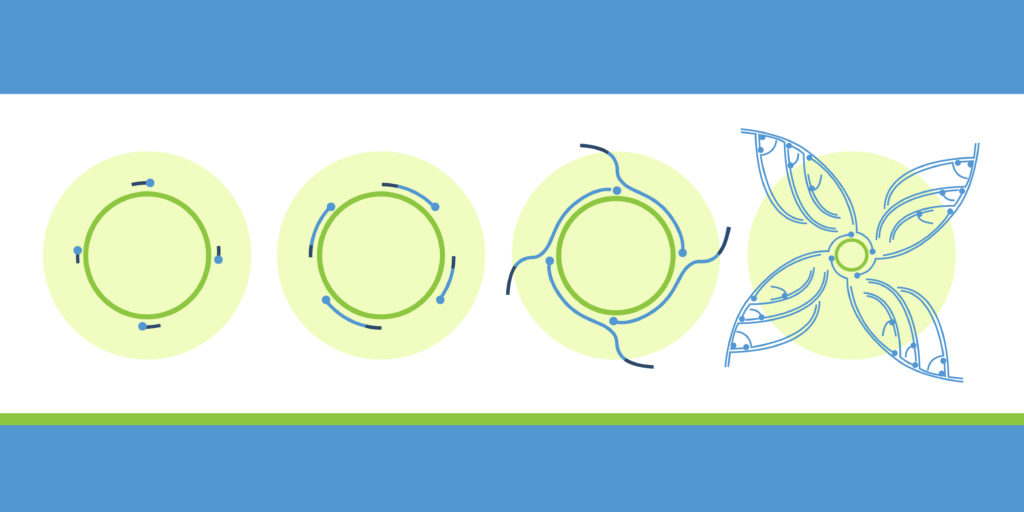
One solution to generating robust quantities of high-quality plasmid from bacterial cells and lysates is rolling circle amplification (RCA), a powerful DNA amplification technique used to generate large quantities of high-quality plasmid DNA and in many situations can replace a miniprep.
One advantage of RCA is that it does not require culturing of bacteria or centrifugation steps to prepare sequencing template: RCA amplifies circular DNA direction from colonies, plagues or microliter volumes of bacterial culture or glycerol stock, and the amplified template is can then be directly sequenced or converted to an NGS library without need for additional purification steps, which means researchers save on the cost of commercial purification columns and plates. And because the RCA amplification process is isothermal, the reaction can be performed in an oven, heat block, water bath or thermocycler.2
First developed in the late 1980s, RCA has since become one of the most widely used methods for amplifying DNA. Because RCA does not require a template DNA strand, it is used for applications such as sequencing and genotyping. In addition, RCA can be used to generate large amounts of DNA for other purposes, such as cloning or transgenesis.
RCA is often used in situations where traditional PCR is not possible, such as when researchers need to amplify large fragments of DNA or RNA. RCA can also be used to generate high levels of template DNA or RNA for sequencing or other downstream applications. The basic principle behind RCA is simple: a circular piece of DNA or RNA is incubated with a set of primers, enzymes, and nucleotides. The enzymes then proceed to “roll” the circle of template DNA or RNA, replicating it many times over. This results in a large amount of template DNA or RNA that can be used for further analysis. Although RCA is a relatively straightforward technique, it has revolutionized the field of molecular biology and has countless applications in research and industry.
Scalable, True Multiplexed Library Prep Enables High-Performance, High-Throughput Plasmid Sequencing
While RCA is a versatile tool for amplifying and preparing large numbers of plasmid samples for downstream sequencing, for NGS-based workflows there is still a need for a fast and scalable approach to convert RCA products to instrument-ready NGS libraries. As discussed in previous blog posts, the plexWell NGS library technology is well-suited to applications with a high need for multiplexing. The use of RCA with multiplexed plexWell library preparation kits is a powerful combination, making NGS sequencing more cost effective by cutting down on the number of labor-intensive procedures and steps needed to generate data from large numbers of plasmids.
The plexWell library prep library technology utilizes a unique, sequential, transposase-based tagging method to general auto-normalized NGS libraries in true multiplexed fashion. This approach is particularly powerful in high throughout sequencing of sequencing of samples with relatively low sequence complexity (e.g., long PCR products, plasmids, and small genomes), where is it a challenge to balance library construction capacity and cost with available sequence capacity.1
One difficulty that comes with high-throughput library prep is normalizing each sample both before and after the workflow to ensure equal read depth across samples. Here, the combination of RCA and plexWell has two important advantages. First, when run to endpoint RCA often has an inherent normalizing property that will generate consistent amount of yield of product based on the amounts of reagent present in the RCA reaction. Second, plexWell kits eliminate much of the need for individual input DNA purification and sample normalization via normalizing transposase-based chemistry. By combining RCA and plexWell, researchers can go from colonies on a plate to NGS verified plasmids in just two days.
Conclusion
Rolling Circle Amplification is a versatile and rapid technique for generating large quantities of plasmid DNA, making it an ideal choice for high-throughput applications. When used with plexWell library preparation kits, this technique saves both time and money because it accelerates a wide-range of plasmid-based sequencing workflows in synthetic biology, cell and gene therapy research, and screening applications.
Have you tried using Rolling Circle Amplification in your own research? Contact us to let us know how it went!
