Introduction
As CRISPR and other genome editing tools enter the clinic, the ability to accurately and reproducibly measure off-target edits is becoming even more critical. In their 2024 guidance, the FDA recommends using multiple methods to measure off-target editing events, including genome-wide analysis.1
In this blog we review different off-target analysis approaches – including in silco, biochemical, cellular and in situ methods, and explore the pros and cons of specific assays like GUIDE-seq3, CHANGE-seq4, UDiTaS5 and DISCOVER-seq6.
The Need for Off-Target Analysis
CRISPR is one of many genome-editing tools that allow targeted modification of DNA. Guide RNA (gRNA) that are complementary to the DNA sequence being targeted direct the Cas9 nuclease to where it should introduce a double-strand break. This break activates the cell’s natural repair mechanisms, that can result in single nucleotide changes, insertions/deletions – either small or large in size – or translocations. Although gRNA directs Cas9 enzyme activity, it is possible the enzyme inadvertently cuts DNA at off-target locations. Addressing these off-target effects is a key area of research to improve gene editing safety and reliability for therapeutic use.
The Beginning of FDA Guidance
In December of 2023, the FDA approved the first CRISPR-based therapy, exa-cel (exagamglogene autotemcel, CASGEVY™)7, for the treatment of sickle cell disease. Exa-cel enables fetal hemoglobin (Hbf) production in adult CD34+ hematopoietic stem and progenitor cells (HSPC) isolated from patients suffering from sickle cell disease. During normal development BCL11A suppresses the production of Hbf. By disrupting the BCL11A gene using CRISPR, suppression is released, allowing Hbf production.
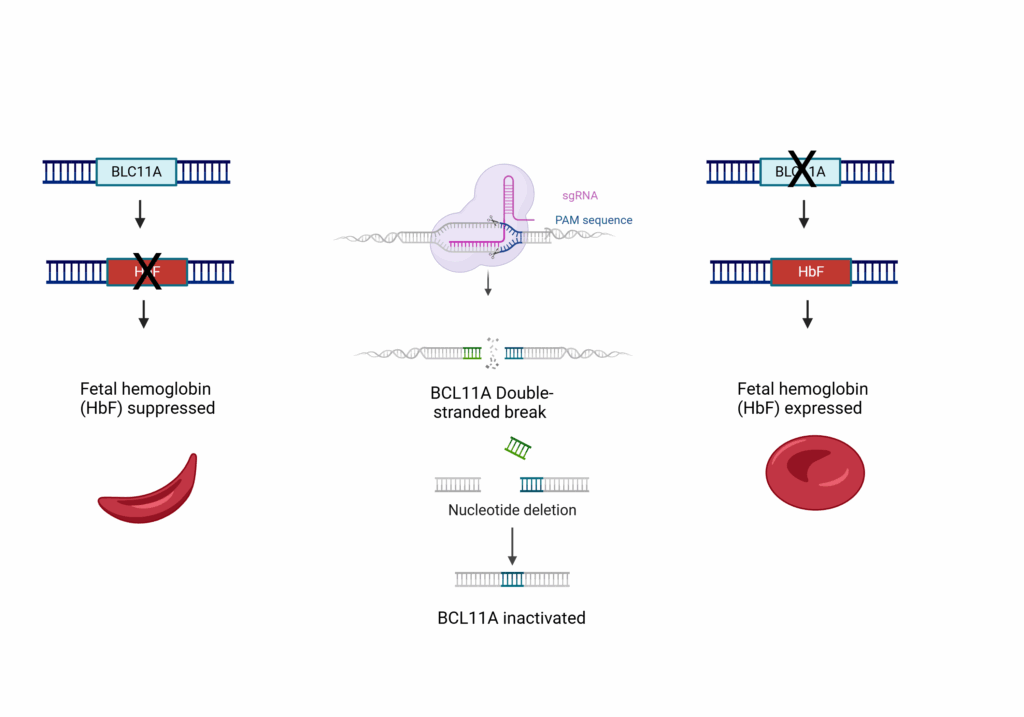
Figure 1. Sickle cell patients produce abnormal hemoglobin S (HbS), which makes red blood cells less efficient at carrying oxygen. In adult cells, the expression of fetal hemoglobin (HbF), which could overcome HbS insufficiencies, is suppressed by BCL11A. Using CRISPR to inactivate BCL11A in hematopoietic stem cells restores expression of Hbf leading to reversal of sickle cell symptoms. Figure created with BioRender.com.
Exa-cel’s success has spurred the development of many more CRISPR-based products that are rapidly advancing to clinical trials, particularly those targeting known genetic disorders and cancer. Despite its promise, however, CRISPR still faces headwinds for widespread use due to concerns of off-target effects and overall safety.
Evolving Off-Target Analysis to Align with Clinical Needs
Investigation of exa-cel off-target activity relied on a commonly used approach in which in silico-predicted sites of homology are amplified and sequenced to identify any non-specific cleavage. During the approval of exa-cel, FDA reviewers flagged two potential shortcomings in this approach2. First, they questioned whether the database that was used adequately reflected the genetics of people of African descent, a target population of exa-cel. Second was the concern that only 40 patients were tested.
Because of the importance of off target effects and the limitation of database searches – often referred to as biased assays because they rely on a priori knowledge – genome-wide off-target studies, or unbiased assays, may be beneficial, especially during pre-clinical studies rather than waiting until clinical trials. Additional benefits are derived if studies such as these are conducted using cells similar to the target cells to improve physiological relevance.
The Need for Standardization
Currently, there is no assay(s) recognized as the gold standard for measuring off-target gene editing activity. Groups, such as the NIST (National Institute of Standards and Technology) Genome Editing Program, are focused on developing reference materials, standardized assays, and best practices that will allow researchers to more effectively and efficiently evaluate the fidelity, safety, and reproducibility of gene therapies. Read our blog entitled ‘Gaining Confidence in Gene Editing Analysis’.
Until the gene editing field adopts a set of scientific norms concerning off-target analysis, scientists and clinicians have the onerous task of choosing both the general approach (i.e. biased versus unbiased) and the specific assay they use to assess off-target effects.
Approaches to Identify CRISPR Off-Target Effects
A variety of assays have been published8,9 to examine off-target activity, each having its own strengths and weaknesses, using one of four general approaches: in silico tools (biased method), or unbiased genome-wide experimental detection which can be conducted using either a biochemical, cellular, or in situ approach. Individual assays within each approach range in their level of sensitivity, throughput, and workflow complexity, with many of the unbiased protocols culminating in next generation sequencing (NGS).
Different approaches excel within the continuum of off-target evaluation:
- In silico – sgRNA design/prediction
- Biochemical – broad discovery
- Cellular– discovery or validation of biological relevance
- In situ –spatial mapping
Table 1 summarizes their strengths and weaknesses that can help inform decision making. The remainder of this article will review in more depth individual assays with the biochemical and cellular approaches.
| Approach | Assays/Tool | Input Material | Detection in… | Strengths | Limitations |
| In silico | Cas-OFFinder, CRISPOR, CCTop, MIT CRISPR tool | Genome sequence + computational models | Predicted sites (based on sequence similarity, PAM rules, models) | Fast, inexpensive, no lab work; useful for guide design | Predictions only; no chromatin, repair, or nuclease activity captured |
| Biochemical | CIRCLE-seq, CHANGE-seq, SITE-seq, DIGENOME-seq |
Purified genomic DNA | Naked DNA (no chromatin) | Ultra-sensitive; comprehensive; standardized | May overestimate cleavage; lacks biological context |
| Cellular | GUIDE-seq, DISCOVER-seq, UDiTaS | Living cells (edited) | Native chromatin + repair | Reflects true cellular activity; identifies biologically relevant edits | Requires efficient delivery; less sensitive; may miss rare sites |
| In situ | BLISS, BLESS, END-seq, GUIDE-tag | Fixed/permeabilized cells or nuclei | Chromatinized DNA in native location | Preserves genome architecture; captures breaks in situ | Technically complex; lower throughput; variable sensitivity |
Table 1: Summary of off-target analysis approaches.
Biochemical, NGS-based Off-Target Assays
Biochemical methods rely on in vitro assays that use isolated genomic DNA and engineered nucleases to directly map potential cleavage sites without requiring living cells. Assays, such as Digenome-seq10, CIRCLE-seq11, CHANGE-seq4, and SITE-seq12, expose genomic DNA to Cas nucleases under controlled conditions and then enrich and sequence the resulting double-strand breaks. Because they eliminate cellular influences like chromatin structure or repair pathways, biochemical assays are highly sensitive and can reveal a broader spectrum of potential off-target sites than cell-based methods. While they often overestimate editing activity compared to in vivo conditions, these techniques provide valuable first-line data for identifying off-target risks, prioritizing candidate sites for further validation, and improving nuclease design.
|
|
DIGENOME-seq | CIRCLE-seq | CHANGE-seq | SITE-seq |
| General description | Treats purified genomic DNA with nuclease, then detects cleavage sites by whole-genome sequencing | Uses circularized genomic DNA and exonuclease digestion to enrich nuclease-induced breaks | Improved version of CIRCLE-seq with tagmentation-based library prep for higher sensitivity and reduced bias | Uses biotinylated Cas9 RNP to capture cleavage sites on genomic DNA, followed by sequencing |
| Sensitivity | Moderate; requires deep sequencing to detect off-targets | High sensitivity; lower sequencing depth needed compared to DIGENOME-seq | Very high sensitivity; can detect rare off-targets with reduced false negatives | High sensitivity; strong enrichment of true cleavage sites |
| Input DNA | Micrograms of purified genomic DNA | Nanogram amounts of purified genomic DNA | Nanogram amounts of purified genomic DNA | Microgram amounts of purified genomic DNA |
| Enrichment step | None (direct WGS of digested DNA) | Circularization of DNA → exonuclease removes linear DNA, enriching cleavage products | DNA circularization + tagmentation → efficient capture of nuclease cuts | Biotinylated Cas9 binds and pulls down cleaved DNA fragments |
| Reference | Kim et al., Nat Methods 2015 | Tsai et al., Nat Methods 2017 | Lazzarotto et al., Nat Biotechnol 2020 |
Table 2: Summary of biochemical off-target assays. DIGENOME-seq – DIGested GENOME Sequencing; CIRCLE-seq – Circularization for In vitro Reporting of Cleavage Effects by Sequencing; CHANGE-seq – Circularization for High-throughput Analysis of Nuclease Genome-wide Effects by Sequencing; SITE-seq – Selective enrichment and Identification of Tagged genomic DNA Ends by Sequencing
Cellular NGS-based Off-target Assays
Cellular methods assess nuclease activity directly in living or fixed cells, capturing the influence of chromatin structure, DNA repair pathways, and cellular context on editing outcomes. Techniques such as HTGTS13, DISCOVER-seq6, BLESS14, UDiTaS5 and GUIDE-seq3, rely on introducing specialized tags, sequencing adapters, or monitoring endogenous repair proteins to map double-strand breaks as they occur in cells. These assays provide biologically relevant insights by identifying which off-target sites are edited under physiological conditions. While they may have lower sensitivity than biochemical assays and often require efficient delivery of both nuclease and detection reagents, cellular methods are essential for validating the clinical relevance of off-target effects and are particularly valuable in therapeutic development.
| HTGTS | DISCOVER-seq | BLESS | UDiTaS | GUIDE-seq | |
| General description | Captures translocations from programmed DSBs to map nuclease activity | Recruitment of DNA repair protein MRE11 to cleavage sites by ChIP-seq | Labels DSB ends in situ with biotin linkers | Amplicon-based NGS assay to quantify indels, translocations, and vector integration at target loci | Incorporates a double-stranded oligonucleotide at DSBs, followed by sequencing |
| Input DNA | Cellular DNA after nuclease expression | Cellular DNA; ChIP-seq of MRE11 binding | Fixed/permeabilized cells; in situ DNA labeling | Genomic DNA from edited cells (amplicon sequencing) | Cellular DNA from edited, tagged cells |
| Sensitivity | Moderate; dependent on translocation frequency | High; captures real nuclease activity genome-wide | Moderate; detects DSBs but limited by labeling efficiency | High for indels and rearrangements at targeted loci | High sensitivity for off-target DSB detection |
| Detects Translocations | Yes | No | No | Yes | No |
| Detects Indels | No | No | No | Yes | Yes |
| Reference | Frock et al., Nat Biotechnol 2015 | Wienert et al., Science 2019 | Crosetto et al., Nat Methods 2013 | Giannoukos et al., BMC Genomics 2018 | Tsai et al., Nat Biotechnol 2015 |
Table 3: Summary of cellular off-target assays. HTGTS – High Throughput Genome-wide Translocation Sequencing; BLESS – Breaks Labeling, Enrichment on Streptavidin and Sequencing; UDiTaS – Uni-Directional Targeted Sequencing; GUIDE-seq – Genome-wide, Unbiased Identification of DSBs Enabled by Sequencing.
Overcoming Assay Bottlenecks
The biochemical and cellular assays reviewed thus far culminate with NGS read outs. Pre-clinical and translational studies typically examine large sample sets. NGS throughput can become burdensome. Scalability of sequencing pipelines, particularly during NGS library preparation, is an area of concern, often causing a bottleneck. Simple library prep workflows, such as seqWell tagmentation, can significantly streamline library prep, improve lab productivity, and allow cost reductions without sacrificing data quality.
Case study: Evolution of CIRCLE-seq to CHANGE-seq
CIRCLE-seq maps CRISPR off-target activity by circularizing genomic DNA, exposing it to Cas nucleases, and enriching the cleavage products via exonuclease digestion prior to sequencing. This genome-wide assay was first published by Tsai et al. in 2017 in Nature Methods11 and has the benefits of low DNA input requirements and a high level of sensitivity. This assay, however, uses traditional NGS library preparation based on DNA shearing or enzymatic fragmentation and adapter ligation that is not easily scaled to accommodate the required throughput.
To overcome this and other assay limitations, CHANGE-seq was developed in 2020 by Lazzarotto et al. and reported in Nat Biotechnol4. Both CIRCLE-seq and CHANGE-seq assays rely on circular DNA enrichment of nuclease cleavage events; however, CHANGE-seq’s adoption of tagmentation-based library prep greatly improves the ease of automation and overall throughput, while maintaining the high sensitivity and low DNA input aspects of CIRCLE-seq. CHANGE-seq has now become a well-known assay commonly used for biochemical off-target analysis.
Taking Tagmentation to the Cellular Level
Similar to the evolution of CIRCLE-seq to CHANGE-seq, GUIDE-seq – a broadly adopted, genome-wide cellular off-target assay – was recently updated in 2025 to GUIDE-seq2 by incorporating tagmentation to address assay limitations (15). First generation GUIDE-seq library preparation uses genomic DNA shearing and a lengthy tagging method that requires end-repair, adapter ligation, A-tailing, and two rounds of PCR. These limitations constrain first generation GUIDE-seq throughput and precluded its use in large-scale studies.
GUIDE-seq2 now offers a genome-wide, unbiased cellular assay with the sensitivity and scalability to support population-scale off-target analysis studies. The highly streamlined library prep workflow has a greatly reduced workflow from one full day down to 3 hours.
Realizing the Promise of Tagmentation
Tn5 for use in tagmentation-based library prep can be purified and loaded with adaptor sequences in research laboratories, however, the process requires multiple days, prior experience and often results in inconsistent loading efficiency and enzyme activity.
Tagify™ custom-loaded transposase reagents from seqWell are available for use in for tagmentation-based NGS library preparation supporting simple, scalable, and reliable gene editing QC analysis. Tn5 or TnX next-generation transposases come pre-loaded with adaptor sequences and fully QC’d to ensure assay reproducibility. Tagify reagents support a diversity of applications including GUIDE-seq2, UDiTaS™, ATAC-Seq, SHARE-Seq, GUIDE-tag, CHANGE-Seq, TTIS-Seq, or RGen-Seq.
Contact us to learn how we can improve your gene editing QC workflow.
Read our Additional Blogs on Gene Editing QC
Excellent Reviews of Assay Methods
- Guo, C., Ma, X., Gao, F., & Guo, Y. (2023). Off-target effects in CRISPR/Cas9 gene editing. Front Bioeng Biotechnol, 11:1143157.
- Atkins, A., Chung, C. H., Allen, A. G., Dampier, W., Gurrola, T. E., Sariyer, I. K., Nonnemacher, M.R., & Wigdahl, B. (2021). Off-target analysis in gene editing and applications for clinical translation of CRISPR/Cas9 in HIV-1 therapy. Front Genome Ed, 3, 673022.
References
- FDA Guidance https://www.fda.gov/media/156894/download
- From Ledford, H. (2023). Is CRISPR safe? Genome editing gets its first FDA scrutiny. Nature, 623(7986), 234-235.
- Tsai, S. Q., Zheng, Z., Nguyen, N. T., Liebers, M., Topkar, V. V., Thapar, V., Wyvekens, N., Khayter, C., Le, L.P., Aryee, M. & Joung, J. K. (2015). GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol, 33(2), 187–197.
- Lazzarotto, CR, Malinin, NL, Li, Y, Zhang, R, Yang, Y, Lee, G, Cowley, E, He, Y, Lan, X, Jividen, K, Katta, V, Kolmakova, NG, Petersen, CT, Qi, Q, Strelcov, E, Maragh, S, Krenciute, G, Ma, J, Cheng, Y, & Tsai, S. Q. (2020). CHANGE-seq reveals genetic and epigenetic effects on CRISPR-Cas9 genome-wide activity. Nat Biotechnol, 38(11), 1317-1327.
- Giannoukos, G, Ciulla, DM, Marco, E., Abdulkerim, HS, BarreraLA, Bothmer, A, Dhanapal, V, Gloskowski, SW, Jayaram, H, Maeder, ML, Skor, MN, Wang, T, Myer, VE & Wilson, CJ. (2018). UDiTaS™, a genome editing detection method for indels and genome rearrangements. BMC Genomics, 19(1), 212.
- Wienert, B., Wyman, S. K., Richardson, C. D., Yeh, C. D., Akcakaya, P., Porritt, M. J., Morlock, M., Vu, J.T., Kazane, K.R., Watry, H.L., Judge, L.M., Conklin, B.R., Maresca, M., & Corn, J. E. (2019). Unbiased detection of CRISPR off-targets in vivo using DISCOVER-Seq. Science, 364(6437), 286–289.
- Philippidis, A. (2024). CASGEVY Makes History as FDA Approves First CRISPR/Cas9 Genome Edited Therapy. Hum Gene Ther. 35(1-2):1-4.
- Guo, C., Ma, X., Gao, F., & Guo, Y. (2023). Off-target effects in CRISPR/Cas9 gene editing. Front Bioeng Biotechnol, 11:1143157.
- Atkins, A., Chung, C. H., Allen, A. G., Dampier, W., Gurrola, T. E., Sariyer, I. K., Nonnemacher, M.R., & Wigdahl, B. (2021). Off-target analysis in gene editing and applications for clinical translation of CRISPR/Cas9 in HIV-1 therapy. Front Genome Ed, 3, 673022.
- Kim, D., Park, J, Bae, S, Kim, E., Kim, S, Yu, HR, Hwang, J, Kim, JI, & Kim, J. S. (2015). Digenome-seq: genome-wide profiling of CRISPR-Cas9 off-target effects in human cells. Nat Methods, 12 (3), 237–243.
- Tsai, S. Q., Nguyen, N. T., Malagon-Lopez, J., Topkar, V. V., Aryee, M. J., & Joung, J. K. (2017). CIRCLE-seq: a highly sensitive in vitro screen for genome-wide CRISPR-Cas9 nuclease off-targets. Nat Methods, 14(6), 607–614.
- Cameron, P, Fuller, CK, Donohoue, PD, Jones, BN, Thompson, MS, Carter, MM, Gradia, S, Vidal, B, Garner, E, Slorach, EM, Lau, E, Banh, LM, Lied, AM, Edwards, LS, Settle, AH, Capurso, D, Llaca, V, Deschamps, S, Cigan, M, Young, JK, & May, A.P. (2017). Mapping the genomic landscape of CRISPR-Cas9 cleavage. Nat Methods, 14(6), 600–606.
- Frock, R. L., Hu, J., Meyers, R. M., Ho, Y. J., Kii, E., & Alt, F. W. (2015). Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat Biotechnol, 33(2), 179–186.
- Crosetto, N., Mitra, A., Silva, M. J., Bienko, M., Dojer, N., Wang, Q., Karaca, E, Chiarle, R, Skrzypczak, M, Ginalski, K, Pasero, P, Rowicka, M, & Dikic, I. (2013). Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat Methods, 10(4), 361–365.
- Lazzarotto, CR, Li, Y, Flory, AR, Chyr J, Yang, M, Katta, V, Urbina, E, Lee, G, Wood, R, Matsubara, A, Rashkin, SR, Ma, J, Cheng, Y, & Tsai, SQ. (2025). Population-scale cellular GUIDE-seq-2 and biochemical CHANGE-seq-R profiles reveal human genetic variation frequently affects Cas9 off-target activity. bioRxiv10.637517; doi: https://doi.org/10.1101/2025.02.10.637517